Freeze Dryers for R&D and Clinial Trial purposes
For the production of small batches
High capacity Freeze Dryers
For the production of large batches
Depending on your requirements, we can provide manual, semi- or fully automated loading systems that go with your freeze dryer. The fully automated loading solution will interface with your filling line and capping line. SERAIL-TECH Engineering has been working with the biggest brands on the market for many years.
We protect your operators and/or your products during loading & unloading of the freeze dryer by providing isolation systems like laminar flow, RAB’S (Restricted Access Barrier System) or isolators.
For more than 50 years, SERAIL-TECH Engineering are committed to design equipment with ever increasing efficiency to the pharmaceutical and biotechnology industries.
We qualify our products taking into account the required specificities, the regulatory framework and the compatibility with tomorrows products.
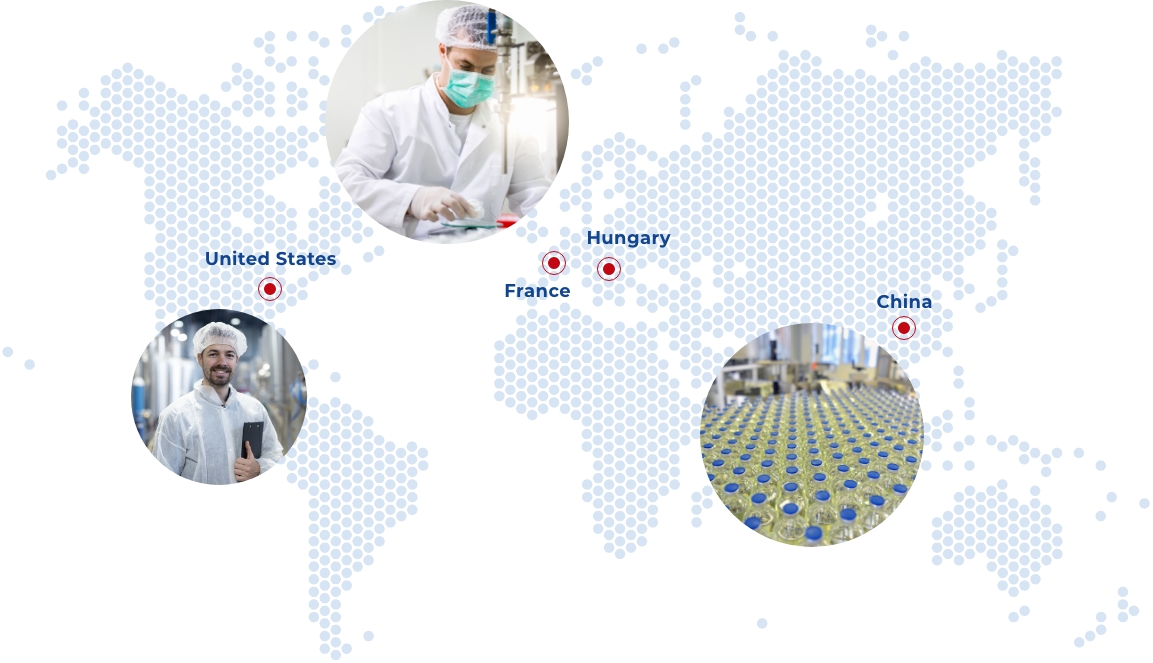
With a direct presence in France, the United States, China and Hungary, we also rely on a large network of partners around the world in order to be as close as possible to the expectations of our customers.
Our teams consist of specialists such as refrigeration mechanics, electricians, automation engineers and process experts.
From the initial project planning and implementation of your requirements through on-site SAT and staff training, our team of experts will be at your side.
Design of a Refrigeration system using the UnifuidTM technology.
Validation of the first CIP system using columns rotating at 180°

Delivery of a trayless loading system under LAF (Laminar Air Flow) to a US customer.

Installation of a SCADA system fully compliant with 21 CFR Part 11.

Installation of a High speed In-line loading system (750 vials / min) under 4 x 30m² isolators.
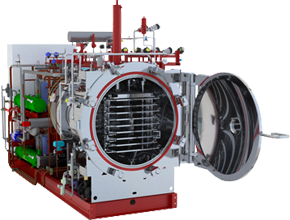
Ultra-fast freezing system on shelves, optimized by an LN2 group, for sensitive products.
Development of hybrid refrigeration systems (Water / Air) for energy saving.
ISerail join forces with a manufacturer of isolation solutions
Launch of the "all-in-one freeze dryer" including the latest SCADA update
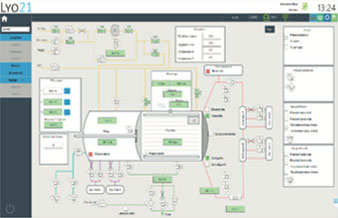
ALUS virtal trays: Concept, design and development
Refrigeration systems at GWP 0 (Global Warming Power)